Solar Energy and Energy Storage Blog
Graphene supercapacitors
Graphene is a thin layer of pure carbon, tightly packed and bonded together in a hexagonal honeycomb lattice. It is widely regarded as a wonder materialbecause it is endowed with an abundance of astonishing traits: it is the thinnest compound known to man at one atom thick, as well as the best known conductor. It also has amazing strength and light absorption traits and is even considered ecologically friendly and sustainable as carbon is widespread in nature and part of the human body.
Graphene is often suggested as a replacement for activated carbon in supercapacitors, in part due to its high relative surface area (which is even more substantial than that of activated carbon). The surface area is one of the limitations of capacitance and a higher surface area means a better electrostatic charge storage. In addition, graphene based supercapacitors will utilize its lightweight nature, elastic properties and mechanical strength.
A Graphene supercapacitor is said to store almost as much energy as a lithium-ion battery, charge and discharge in seconds and maintain all this over tens of thousands of charging cycles. One of the ways to achieve this is by using a a highly porous form of graphene with a large internal surface area (made by packing graphene powder into a coin-shaped cell and then dry and press it).
What are supercapacitors?
Supercapacitors, also known as EDLC (electric double-layer capacitor) or Ultracapacitors, differ from regular capacitors in that they can store tremendous amounts of energy.
A basic capacitor usually consists of two metal plates, separated by an insulator (like air or a plastic film). During charging, electrons accumulate on one conductor and depart from the other. One side gains a negative charge while the other side builds a positive one. The insulator disturbs the natural pull of the negative charge towards the positive one, and that tension creates an electric field. Once electrons are given a path to the other side, discharge occurs.
Supercapacitors also contain two metal plates, only coated with a porous material known as activated carbon. They are immersed in an electrolyte made of positive and negative ions dissolved in a solvent. One plate is positive and the other is negative. During charging, ions from the electrolyte accumulate on the surface of each carbon-coated plate. Supercapacitors also store energy in an electric field that is formed between two oppositely charged particles, only they have the electrolyte in which an equal number of positive and negative ions is uniformly dispersed. Thus, during charging, each electrode ends up having two layers of charge coating (electric double-layer).
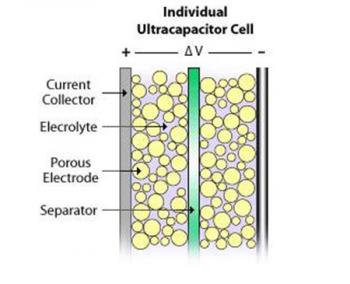
Batteries and Supercapacitors
Unlike capacitors and supercapacitors, batteries store energy in a chemical reaction. This way, ions are inserted into the atomic structure of an electrode, instead of just clinging to it like in supercapacitors. This makes supercapacitors (and storing energy without chemical reactions in general) able to charge and discharge much faster than batteries. Due to the fact that a supercapacitor does not suffer the same wear and tear as a chemical reaction based battery, it can survive hundreds of thousands more charge and discharge cycles.
Supercapacitors boast a high energy storage capacity compared to regular capacitors, but they still lag behind batteries in that area. Supercapacitors are also usually more expensive per unit than batteries. Technically, it is possible to replace the battery of a cell phone with a supercapacitor, and it will charge much faster. Alas, it will not stay charged for long. Supercapacitors are very effective, however, at accepting or delivering a sudden surge of energy, which makes them a fitting partner for batteries. Primary energy sources such as internal combustion engines, fuel cells and batteries work well as a continuous source of low power, but cannot efficiently handle peak power demands or recapture energy because they discharge and recharge slowly. Supercapacitors deliver quick bursts of energy during peak power demands and then quickly store energy and capture excess power that’s otherwise lost. In the example of an electric car, a supercapacitor can provide needed power for acceleration, while a battery provides range and recharges the supercapacitor between surges.
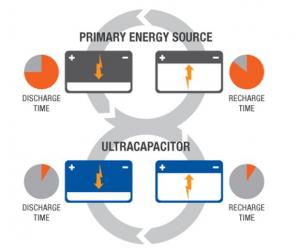
Common supercapacitor applications
Supercapacitors are currently used to harvest power from regenerative braking systems and release power to help hybrid buses accelerate, provide cranking power and voltage stabilization in start/stop systems, backup and peak power for automotive applications, assist in train acceleration, open aircraft doors in the event of power failures, help increase reliability and stability of the energy grid of blade pitch systems, capture energy and provide burst power to assist in lifting operations, provide energy to data centers between power failures and initiation of backup power systems, such as diesel generators or fuel cells and provide energy storage for firming the output of renewable installations and increasing grid stability.
Rivaling materials
Several materials exist that are researched and suggested to augment supercapacitors as much (or even more than) graphene. Among these materials are: hemp, that was used by Canadian researchers to develop hemp fibers that are at least as efficient as graphene ones in supercapacitor electrodes, Cigarette filters, which were used by Korean researchers to prepare a material for supercapacitor electrodes that exhibits a better rate capability and higher specific capacitance than conventional activated carbon and even higher than N-doped graphene or N-doped CNT electrodes.
Graphene supercapacitors commercialization
Graphene supercapacitors are already on the market, and several companies, including SuperCaps, Skeleton Technology, the CRRC, ZapGoCharger, and Angstron Materials are developing such solutions.
New study commissioned by EUON assesses graphene’s potential impact on health and environment
The European Union Observatory for Nanomaterials (EUON) has published a study that has systematically reviewed and critically assessed the potential health and environmental effects of graphene, graphene oxide, and other two-dimensional (2D) materials, based on existing public information of the last ten years.
It was explained that graphene’s rising popularity and broad application potential raise the need for more efforts in researching its safety aspects. The current study identified potential risks in specific scenarios, for example for surface water and soil located near point sources (such as production sites).
The study, conducted by Innovamol Consulting Srl, Modena (Italy), concludes that cytotoxic effects have been identified for specific graphene and 2D materials both on health and environment. The study report recommends that when health and environmental risks are reported or identified for a specific graphene or 2D material, doses and exposure scenarios should be considered for their manipulation and use.
According to the report, the toxicity and ecotoxicity of graphene-based materials deserve more research efforts using OECD test guidelines and ISO documentary standards. When reporting the results of these tests, a precise description of the material used in the tests is required to enable more specific conclusions on toxicity and ecotoxicity, which currently cannot be generalized.
(euon.echa.europa.eu) | Dec 10,2022 by Roni Peleg
If you’re a regular viewer of vaguely science-based YouTube channels like this one then you’ll probably know only too well that we’re living in a rapidly changing world where some bright spark somewhere on the planet is coming up with a technology or device that gets hailed as a game changer or revolutionary breakthrough almost every week.
Most of them don’t end up changing any games or starting any revolutions though, but every now and then science hits the jackpot and the world takes another technological leap forward. Way back in 1959 the American physicist Richard Feynman gave a talk in which he described his vision for one of these winners.
It was a process that would allow scientists to manipulate and control individual atoms and molecules. That might not sound earth-shattering to our 21st century ears but it was pure science fiction back then.
Feynman’s dream became a reality in the 80s though with the advent of something called the scanning tunneling microscope for which its inventors, Gerd Binning and Heinrich Rohrer received the Nobel prize for physics in 1986.
That invention allowed scientists to do precisely what Feynman had envisaged more than two decades earlier and the world got a fancy new word to add to the dictionary… nanotechnology.
Since then nanotechnology has genuinely revolutionized almost every aspect of our modern day lives from pharmaceutical products to construction materials and industrial processes through to fuel efficiency and even the clothes we wear.
And now nanotechnology has reached the world of electric vehicle batteries and needless to say it’s gonna be a game changer! Hello and welcome to Just Have a Think. So we know that nanotechnology is to do with atomic and molecular scale stuff, which means we’re talking small right? I mean really small.
A nanometer is a billionth of a meter. A human hair is about a hundred thousand nanometers wide so the ability to manipulate stuff at nanoscale is pretty spectacularly smart science. And it turns out when you get down to that kind of microscopic level weird stuff happens.
Atoms start reflecting light differently for example, so gold can become purple and silver can become yellow “and your mommy suddenly becomes your daddy, and everything looks like a giant cupcake”.
Perhaps more significantly, getting down to Nanometer scale allows scientists to vastly increase the surface area of a material which means more atoms can interact with atoms of other materials.
And then all sorts of benefits start kicking in. You get materials that are stronger, more durable and even more electrically conductive as a result. According to the US National nanotechnology initiative “Nanotechnology is not simply working at even smaller dimensions.
Rather working at nanoscale enables scientists to utilize the unique physical chemical mechanical and optical properties of materials that naturally occur at that scale.” The potential of nanotechnology certainly hasn’t escaped the attention of the auto industry and in particular the rapidly developing electric vehicle sector which has spent years and years looking for ways to reduce weight and improve the performance of vehicle batteries.
Much has been promised by so many but as is so often the case these days it’s a Chinese company that’ll be the first to launch an actual vehicle onto the actual market that actually contains nanotechnology in its actual battery.
They’re called GAC group and they’re about to launch the latest version of their Aion V crossover SUV featuring lithium-ion batteries enhanced with graphene, which is a nanomaterial that China is particularly obsessed with.
Specifically GAC’s technology uses graphene instead of graphite as the anode material. Graphite’s the usual choice for lithium-ion battery anodes because it easily transfers collected electrons into the metal wires of an electrical circuit but it does have its limitations.
It takes six carbon atoms in graphite to hold onto a single lithium ion. That relative weakness limits how much lithium the electrode can hold onto which in turn dictates how much potential energy the battery can store.
Studies over many years have shown that unlike a lump of graphite a graphene nanosheet can absorb lithium ions on both faces of the sheet and along its edges and even in any defect sites. That gives it a theoretical capacity of 744 milliamp hours per gram compared with only 372 milliamp hours per gram for graphite.
That’s twice the capacity which is really very significant indeed. The main limiting factor until recently has been cost. At several hundred dollars per gram, graphene is still a premium product.
Until we find a way to mass produce it in a more economical way it’ll probably be limited in its real world applications. But GAC group have come up with a technology called 3DG or 3-dimensional graphene, which they reckon reduces the graphene cost in their lithium ion battery anode by a factor of 10, which brings graphene firmly into the realms of feasible production budgets.
GAC say its graphene based battery has a 6C fast charge capability. In other words it’s capable of receiving 6 times its total capacity in an hour, which is significantly higher than any existing EV on the market today.
Charge and discharge rates in batteries are often expressed using this C rate figure. The higher the number the greater the charge the battery can accept for its given capacity. The downside with very high C rates is that they tend to generate high temperatures which can quickly degrade the materials inside the battery.
And that’s really one of the main limitations holding back the development of super fast charging for EVs. Graphene is much better at accepting those higher charges without degradation though, and although GAC haven’t provided specific technical details of how their 3DG tech works, it’s quite likely that it’s this property of graphene that’s allowed them to achieve such high charge rates.
The result is the new Aion V can be recharged from zero to 80 percent in just eight minutes. That’s getting much closer to the time it takes to fill up an internal combustion engine car with a tank of fuel.
GAC aren’t the only ones to have embraced a nanotechnology solution for their battery tech though. Mercedes-Benz recently announced a joint partnership with the U.S company Sila Nanotechnologies, to develop a battery for their G-class SUV that replaces the graphite in the anode with silicon nanoparticles.
The principle is not dissimilar to the graphene that GAC have used. A silicon atom can bind to four lithium ions which in principle means a silicon based anode could store 10 times as much energy as a graphite anode.
Silicon is very cheap and very abundant so there’s a big commercial advantage there over graphene. The trouble is as the battery charges up all those lovely lithium ions rush in to bind with the silicon and the anode swells up by as much as 300 percent and then it shrinks back down again as the battery discharges.
That’s an awful lot of movement fatigue and it means most developmental silicon anodes quickly fracture and break down making the battery useless. But once again the weirdness of nanomaterials has come to the rescue.
They have much higher percentage of their atoms at the surface relative to the number of atoms in their interior and because those surface atoms have fewer atomic neighbours locking them in place they can move much more easily to respond to the stresses and strains of expansion and contraction.
It’s a bit like why aluminium foil can be bent and scrunched up very easily compared to a thick lump of the same material. Sila’s silicon nanotechnology is, they tell us, the result of more than 10 years of chemical research and more than 55 000 iterations, the result of which is a battery with an energy density of more than 800 watt hours per litre.
Now I don’t know about you but I’m more used to reading about battery energy in terms of watt hours per kilogram so I had a quick look at Battery University online and it explains that specific energy or gravimetric energy density defines battery capacity in weight, which is indeed watt hours per kilogram, but energy density or volumetric energy density reflects volume in litres.
Whichever metric you choose to focus on though, the important thing is the relative performance between different battery chemistries. Sila’s website provides this chart showing the limit of today’s lithium-ion batteries and how their technology will vastly improve things in the coming years.
Mercedes-Benz are aiming to launch their new G-Class range with as much as 40 percent more battery energy density as soon as 2024 and I reckon we can expect to see a whole raft of other manufacturers following suit very quickly.
And as an added bonus Sila reckon these batteries will be manufactured using 100% renewable energy at their brand new Washington state facility. I think it’s fair to say we’re still very much in the wild west territory of the electric vehicle development cycle.
New chemistries and concepts are popping up all the time, all claiming to be revolutionary game changers of course! Will you be charging your EV in under 5 minutes in 10 years time or simply topping it up as you drive along via inductive charging plates under the road surface? Or perhaps you’ll simply drive into a kiosk when your battery is dead and wait while a robot swaps it out for a fully charged one.
Maybe we’ll end up filling our cars with hydrogen instead and perhaps we won’t even own our own vehicle in the coming decades and we’ll just tap on an app and wait two minutes for an autonomous vehicle to arrive and take us to our destination.
All of these technologies already exist. It’s just a matter of which one gains market supremacy the fastest. For someone like me who is now well into middle age it feels like the world has changed beyond recognition since I learned to drive in a crappy old Nissan Micra more than three decades ago, but based on what’s going on in the tech world right now, hold on to your hats folks because you ain’t seen nothing yet! No doubt you’ve got views about these rapidly developing technologies.
If you do, or if you work in the industry and you can share some insights with us, then why not jump down to the comments section below and leave your thoughts there. That’s it for this week though.
A massive thank you as always to our fantastic Patreon supporters who keep these videos completely independent and ad free. And a special thank you to the folks whose names are scrolling up the screen here, all of whom reached anniversaries of Patreon support in June.
If you feel like you could support the channel for about the price of a coffee each month via Patreon then you’ll get access to exclusive content from me and you’ll be able to suggest future videos in monthly content polls.
And you can find out how to do all that by visiting patreon.com/justhaveathink And of course the best and easiest way you can support the channel via YouTube is by clicking that subscribe button and hitting the notification bell.
It’s completely free and dead easy to do, you just need to click on the little icon in the corner or on that icon there. As always thanks very much for watching, have a great week, and remember to just have a think.
See you next week.
TOD/TOU pricing
Micro-Grid Energy Systems
What is a Micro-Grid What is a Micro-Grid? A micro-grid is an innovative energy system that operates independently or in conjunction with the main power
solarkits
Battery KITS Safari LiON LT 500 Watt All Purpose Solar Backup Generator SALE: Save 15% LiON Safari ME 2000 watt Heavy Duty Solar Backup Generator SALE: Save
shed
Solar for Sheds (standalone off-grid) Solar Sheds usually fall under the category of DIY systems and can honestly be installed with basic electrical knowledge. There
energy-rates
ENERGY RATE CHANGES will be a major Negative Impact to All Americans! Proposed energy rate changes on electricity including the use of solar energy and
nem3
(Comments in blue are from us… So how do YOU feel about government interference in the market) Solar power industry slams latest proposed California Net Energy Metering Regulations (NEM
answer
Time of Day/Time of Use Utility Pricing, Net Metering, Solar Energy Evolution & Benefits Its OK to be excited about moving to Solar Energy for



